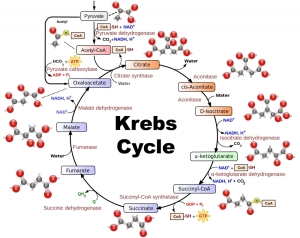
Enzymes are biological catalyst that speeds up the rate of a reaction. A catalyst is a substance which increases the rate of a chemical reaction without itself being used up in the process. Industrial process use catalysts to speed up the production of important chemicals, reducing the need for high temperatures and pressures. An organism’s metabolism consists of thousands of different reactions. Each of these needs different catalysts to enable the reaction to take place. These catalysts called enzymes; hence an enzyme is also an organic catalyst which speeds up the rate of a reaction.
The substance upon which the enzyme acts is called its substrate. Metabolism consists of hundreds of reactions linked together, where the product of one reaction is the substrate for the next. This is known as a metabolic pathway and each step is catalyzed by a different enzyme.
There are thousands of different reactions taking place in living cells; therefore each reaction needs to have a specific enzyme in order to catalyze the reaction. Enzymes are proteins which are composed of sub-units called amino acids that can be linked to each other in an infinite number of different ways. This therefore means that the primary structure of an enzyme shows the unpredictability needed to achieve specificity.
However, this specificity depends on the shape of a small part of the enzyme called the active site, which is the place where the enzyme actually comes into contact with the substrate.
A property of enzyme that makes them important as analytical and research tools is the specificity that they display relative to the reactions that they catalyze. Some enzymes display absolute specificity. This is where the enzyme catalyse only one particular reaction while other enzymes shows relative specificity which is where the enzyme binds to some structurally related substrates. There are 4 types of specificity and they are; absolute specificity, group specificity, stereochemical specificity and last but not least linkage specificity. Absolute specificity is when the enzyme itself will catalyze only one reaction. Group specificity is where the enzyme itself will act on molecules that have detailed functional group for example, amino, phosphate and methyl groups. Linkage specificity, this is where the enzyme acts on a particular type of chemical bond, no matter of what the rest of the molecule structure appears to be. And sterochemical specificity is where the enzyme itself will act on a particular steric or optical isomer.
Non- covalent bonds is responsible for the binding of reversible inhibitors to enzymes. This non-covalent interaction depends upon what the inhibitors bind to, such as, the hydrogen bonds, the hydrophobic interactions or the ionic bonds.
These are referred to as weak bonds and combines to produce strong and specific bonding. There are 3 kinds of reversible inhibitors and they are competitive, non-competitive and uncompetitive inhibitors.
Competitive inhibitor is where the inhibitor and substrate are in a competitive with each other in order to bind to the enzyme at the same time. This can be overcome by simply increasing the substrate concentration level or in other words out-compete the inhibitor. The second type of inhibition is uncompetitive inhibition. These along with the enzyme’s substrate bind to the enzyme at the very same time. However, this binding of the inhibitor affects the binding of the substrate and vice-versa. This type of inhibition is reduced by simply increasing the substrate concentration. The third type of inhibition is non-competitive inhibition. This type of inhibitor reduces the activity by binding to the active site; however, it does not affect the binding of the substrate.
Temperature, substrate concentration as well as pH are all factors that affect enzyme activity. However, in this lab we focused mainly on temperature and substrate concentration. As you increase the temperature it causes the molecules to move faster and this in turn increases the enzyme activity. This means that there will be collision between the molecules and the enzyme. This therefore affects the rate, meaning that the higher collision rate allows an increase in the reaction rate, but, only up to a certain point. If there is still an increase in the temperature, it will cause the enzyme’s protein to start to denature which is a potentially permanent process. The optical temperature ranges of about 25-40 degrees Celsius.
An increase in substrate concentration will also cause the enzyme activity to increase. This is also due to the increase in collisions between the substrate and the active site. However, at some point, if the substrate concentration still increases, there will have no effect on the enzyme activity because all the active sites will be taken up.
REFERENCES:
http://en.wikipedia.org/wiki/Enzyme
http://www.chem4kids.com/files/bio_enzymes.html